

Sodium borohydride is relatively insoluble in ether solvents, so these are rarely used for borohydride reductions. Similar reaction with an alcohol is relatively slow, so the most common reaction solvents for reduction or organic substrates are ethanol and propan-2-ol, although methanol is also used. 27 Sodium borohydride reacts with water to form hydroxyborohydride intermediates, and these products are also mild reducing agents. Water and alcohol solvents are preferred due to the excellent solubility of sodium. 32 The relatively poor reactivity of sodium borohydride is reflected in the solvents used for the reduction.
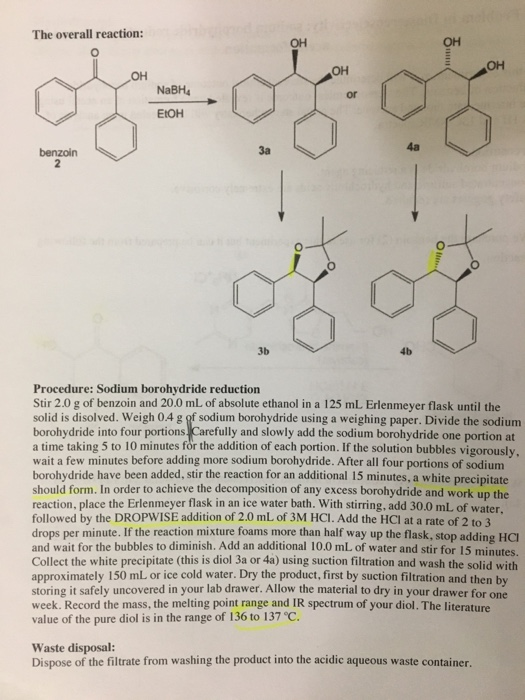
Sodium borohydride is useful for the reduction of aldehydes, ketones, or acid chlorides in the presence of other easily reducible functional groups.
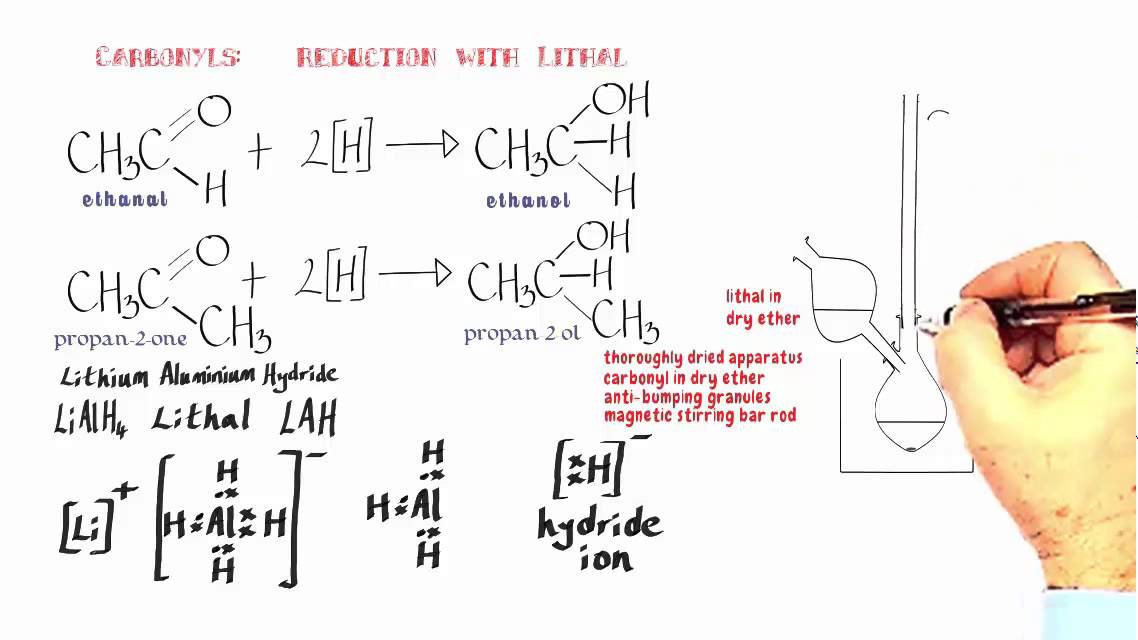
Sodium borohydride is considered to be a selective reagent, 31 which means that it is a weaker reducing agent when compared to LiAlH 4 (e.g., see Section 7.6). 4 N a H + B O M e 3 → NaBH 4 + 3 M e O − N a +


 0 kommentar(er)
0 kommentar(er)
